The Biophotonics and Optical Sciences Facility is a suite of three laboratories located in the Department of Physics & Astronomy. This state-of-the-art facility encompasses approximately 1500 sq. ft. and contains a quantum optics system, multiple optical tweezers systems, and a Laser Tweezer Raman Spectroscopy system.
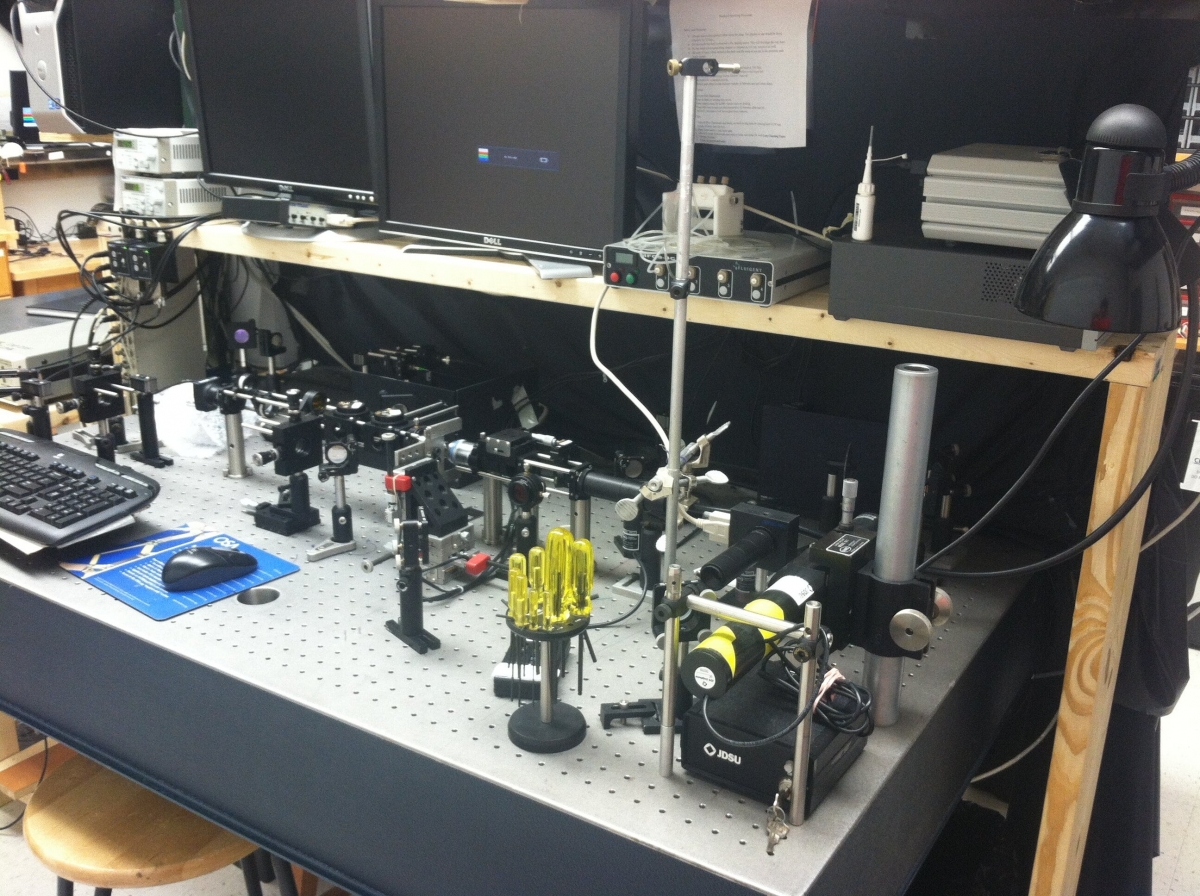
Optical Tweezers Setup
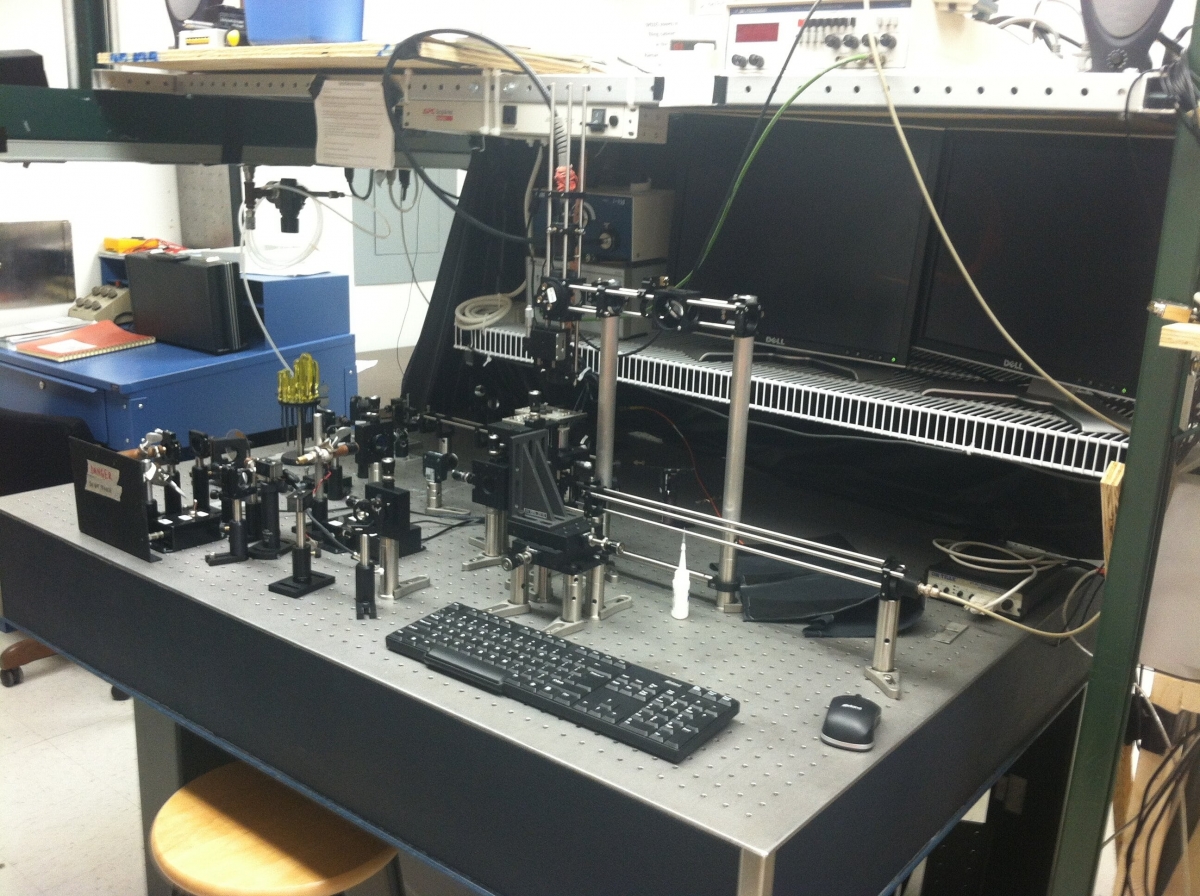
Fluorescence Anisotropy Setup
Optical Tweezers Laboratory
Optical tweezers are a flexible and useful tool for the manipulation of small objects ranging from the nanometer scale to the micrometer scale and have been used to study systems including those at the single molecule level, properties of fluids, systems of colloids, and are also used as tools for manipulation techniques. The optical trap is formed using a tightly-focused laser beam (usually in water or a buffer) which creates an extreme intensity gradient over a small volume capable of trapping a small object which can be manipulated and studied with optical techniques, usually in order to gain information about its motion. One of the more popular applications of optical tweezers is the ability to study the response of a molecule or system of molecules to applied forces. This is accomplished by chemically attaching a bead to a sample, trapping the bead with a laser, then stretching or moving the sample, affecting the attached bead whose position is monitored, and allowing for the determination of mechanical or dynamic properties of the sample material. A position-sensing laser is used to track the motion of the trapped bead. With this position information, the mechanical properties of the microstructure and nearby thermodynamic properties can be determined.
The Optical Tweezers Lab at Appalachian State University houses an Optical Tweezers microscope, a Fluorescence Optical Tweezers microscope, and portable training tables. The research microscopes are both located on a 6ft x 8ft floating optical table. The portable optical breadboards are used to train new students on basic optical principles and also introduces them to components used in the labs before they operate the research equipment.
The Optical Tweezers microscope is a simple custom build microscope. Zeiss 60x objective lens, Thorlabs CMOS camera, beam pickoffs for running laser power detection during operation, automated steering mirrors, 3 axis piezoelectric sample mount. The trap beam is a 975 diode laser that is spilt with a polarizing beam splitter to create two independent and steerable trapping beams. A 632 HeNe gas laser is used for trapped particle position detection. This microscope is used for force microscopy measurements and also tweezers automation projects.
The Fluorescence Optical Tweezers microscope is another custom build system. Zeiss FLUAR 100x 1.3NA objective, Thorlabs CMOS camera, duo-lateral position sensor, B&H SPC-130-EM timing board, 2 fiber coupled APDs. A 650nm diode laser is used for particle optical trapping while a 532nm diode laser allows position sensing. A 405nm diode laser is intensity modulated at high frequencies with a Thorlabs lithium niobate eloctro-optic amplitude modulator. This 405nm laser is used for fluorescence excitation and the modulated intensity is used for frequency domain anisotropy measurements. This microscope is primarily used to study fluorescence of mostly tagged biological samples and other samples where we wish to look at rotational correlations.
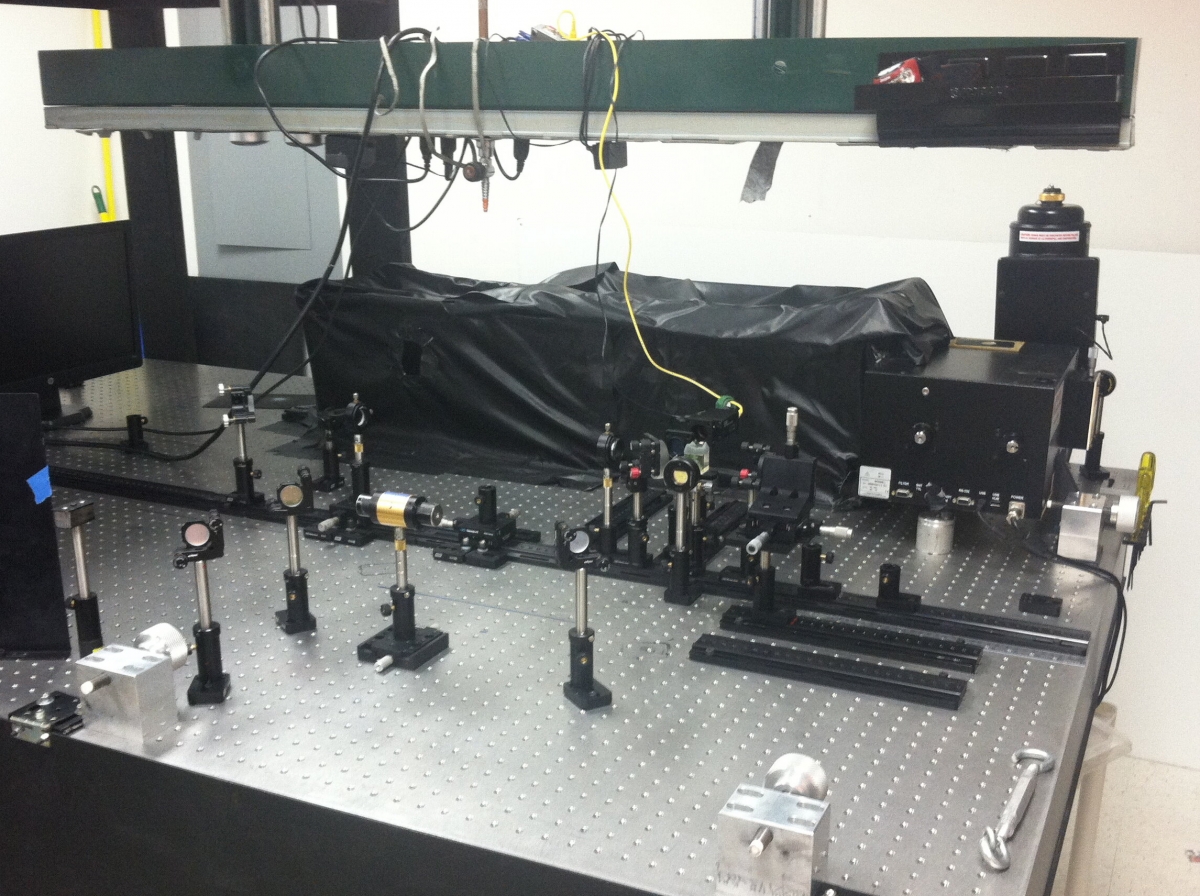
Raman Spectroscopy Setup
Raman Spectroscopy Laboratory
Raman Spectroscopy is a method of studying a sample based on Raman scattering, which is a versatile method commonly used to identify, characterize, and analyze materials. This type of scattering is inelastic, where incident light gains or loses energy due to its interaction with vibrating molecules. The amount of energy gained or lost is directly related to the vibrational energy of the scattering molecule. Since all molecules have different vibrational states, the signal scattered from the sample is unique to every molecule, so a Raman spectrum can be thought of as a molecular fingerprint. Since each distinct molecule has a signature spectrum, Raman spectroscopy can provide a database of signature signals for each distinct type of compound in existence. Raman spectroscopy offers a unique way to non-invasively examine the structure of a material or system.
The Raman Spectroscopy Laboratory at Appalachian State University houses our Raman spectroscopy system. The system is a custom built open-air, tabletop Raman system; using both a 785nm diode laser and an air-cooled 514nm multi-line gas laser. We also have the capability of using fiber optics on the system with both lasers. The system is built on a vibration dampened optical table. We use a back illuminated, deep-depletion, liquid-nitrogen-cooled CCD coupled to a Princeton Instruments Acton SP 2300 spectrometer. The system is designed to be highly versatile by incorporating the open-air set-up, fiber optics, and an optical viewing microscope. The design also allows us to work with samples varying in size from large, bulk samples, to much smaller samples.
Within the Raman Spectroscopy Laboratory, we have an Elan2 liquid nitrogen generator capable of producing 4 liters of liquid nitrogen per day. The liquid nitrogen is used to cool the CCD's in two laboratories and provide liquid nitrogen to other laboratories as needed.
The main function of the Raman Spectroscopy laboratory is to study the structure of inorganic, and biological materials. The laboratory is utilized in many projects, crossing multiple disciplines, including physics, biology, chemistry, and computer science. Some of the past and current research projects being worked on in this facility include, studying E. Coli structure, the structure of biofilms, the detection of microbes in the fermentation process, and predicting pre-term labor with cervical tissue.

Raman Tweezers Setup
Laser Tweezer Raman Spectroscopy Laboratory
A Laser Tweezer Raman Spectroscopy (LTRS) apparatus combines the precision and power of optical tweezers with the diagnostic capabilities of Raman spectroscopy. Our custom-built apparatus uses three lasers: a 785 nm diode laser to obtain the Raman spectra, a 1064 nm diode laser to trap particles, and a 633 nm HeNe laser for position sensing. A microscope was built-in the middle of our system where the sample is placed in order to interact with the light. In order to achieve the optimal conditions required, all three lasers travel through a series of mirrors until they all reach the sample. White light is sent through the sample in the opposite direction of the Raman and the trapping laser beams. The white light is reflected into a visual camera that allows us to observe the sample interaction with the lasers and enables us to trap an individual particle as well as the trap and the trapped object across the sample by changing the position of the trapping laser beam. Once an object has been trapped, the Raman laser is reflected back along a different path until it reaches the spectrometer, which can then take a Raman spectrum of the trapped object. The position-sensing laser also passes through the sample and continues to the position sensing diode, which tells us the location of the trapped object.
The LTRS Lab at Appalachian State University houses the LTRS System along with our chemistry station. The chemistry station consists of a vented fume hood, chemical storage areas with appropriate safety materials, and general basic level equipment. The LTRS system is a custom build microscope system located on a 5ft x 6ft air floated optical bench. The bench and the system are encased in a blackout cage that helps control stray light. The Raman spectroscopy components are a Princeton Instruments Acton SP 2300 spectrometer with a LN2 cooled PyLon CCD. A 785nm diode laser is used for the Raman excitation; a 532 diode laser is used for trapped particle position detection, and a 1064nm diode laser is used as the trapping laser. The oil emersion Zeiss FLUAR 100x 1.3NA objective allows submicron visual resolution with a Thorlabs CMOS camera.
The primary function of the LTRS microscope is to study the structure and function of small aqueous substances. The current research project is the study of bacterial colony structures.